Worth knowing from the area of cleanroom qualification
The knowledge area cleanroom qualification answers questions about the cleanroom classes and the tests within the scope of a cleanroom qualification. You will also receive an overview of the specifications from DIN EN ISO 14644-3:2020-08.
Here you will find answers to the following questions:
- Which cleanroom classes are there?
- Which tests/measurements are carried out during a cleanroom qualification?
- What innovations does DIN EN ISO 14644-3:2020-08 bring with it with regard to the performance of leak tests on HEPA filters?
- What influence do the changes to DIN EN ISO 14644-1 have on the qualification of cleanrooms?
Which cleanroom classes are there?
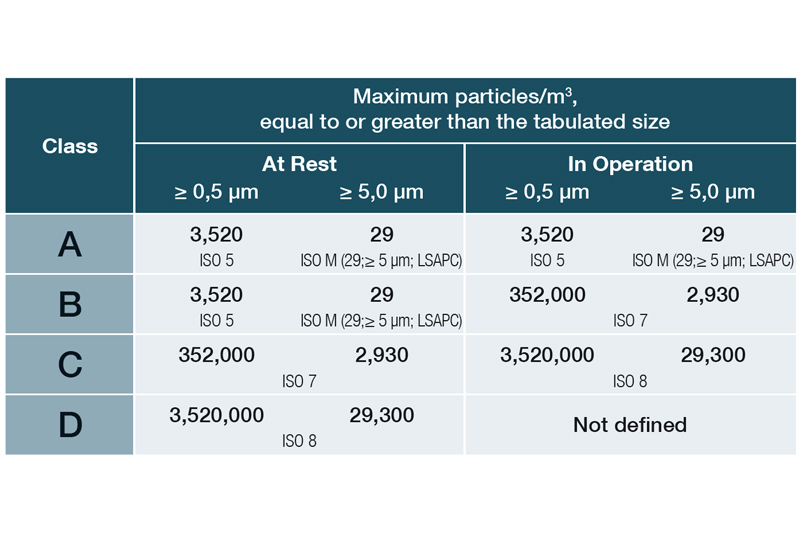
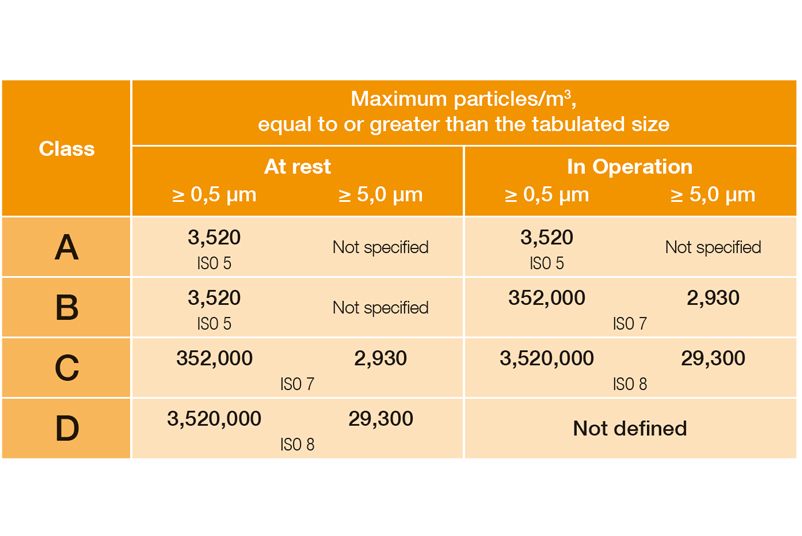
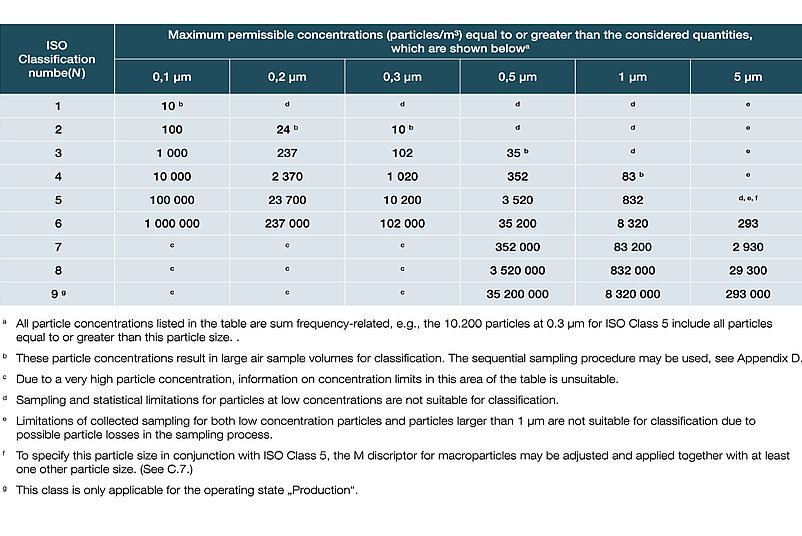
In DIN EN ISO 14644-1, the various cleanroom classes (ISO 1-9) are classified on the basis of the maximum permissible particle concentration (in particles per cubic metre of air). In the EU GMP guidelines, the cleanroom classes are assigned the letters A to D, with an additional distinction being made between dormante state and manufacturing.
Limit values for particles (permanent monitoring) in Annex 1 as of 08/2022:
EU Guidelines to Good Manufacturing Practice, Annex 1, 08/2022
Limit values classification (qualification) in Annex 1 as of 08/2022:
EU Guidelines to Good Manufacturing Practice, Annex 1, 08/2022
Classification of air purity based on particle concentrations in accordance with DIN EN ISO 14644-1:2016:
Which tests/measurements are carried out during a cleanroom qualification?
The following measurements are typically performed during a cleanroom qualification:
- Climate measurements
- Pressure measurements
- Flow measurements
- Determination of the Particulate Purity Class
- Filter Integrity Tests and Measurement of Recovery Times
- Microbiological monitoring
What innovations does DIN EN ISO 14644-3:2020-08 bring with it regarding the performance of leak tests on HEPA filters?
Performing the filter leak test has not become any easier. There is additional formalism (e.g., derivation of Dp) and continued heavy fare in leakage definition via Na. The conformity statement for filters H13/H14 has become more stringent by a factor of 5. This has an impact on the feasibility (more re-measurements) and especially on the aerosol load of the HEPA filters. The concentrations increase several times compared to the previous version. The acceptance criterion of an in-situ test was formulated more strictly than the local transmittance in EN 1822-1. Further aggravation comes from the unnecessary definition of Na/Np. By choosing such a small value for counting events, there will be many counting events.
What changes were introduced in the August 2022 revision of Annex 1 of the EU GMP Guidance for the qualification of cleanrooms?
Despite some changes in the August 2022 version of Annex 1 of the EU GMP Guidance, the basic concept remains unchanged. There are no significant alterations to the recovery time definition, measurement of air velocities in the TAV area, and microbial status. However, a contamination control strategy (CCS) is now required as a central element of quality assurance measures. Additionally, specific requirements for minimum efforts and intervals for qualification measurements are introduced, with an explicit distinction between measurements in the "at rest" and "in operation" operating states. The harmonization of limit values for particle concentration in determining the particulate cleanliness class with DIN EN ISO 14644-1:2016 creates a discrepancy between the now different measures of qualification and monitoring.
Establishment of a GMP monitoring concept in the pharmaceutical manufacturing sector